Facial Paralysis Clinical Trial Results
Data Science and Analytics
Tags and Keywords
Trusted By



"No reviews yet"
Free
About
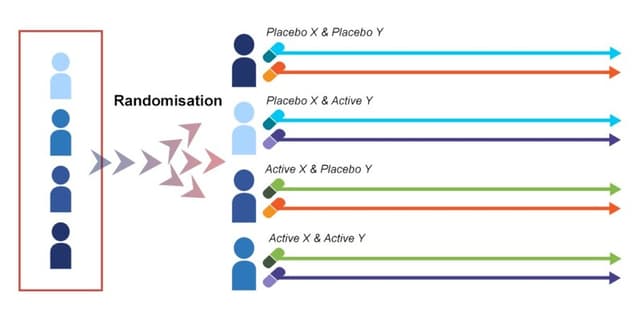
Detailed patient-level clinical trial data derived from a study investigating treatments for Bell's Palsy. This synthetic dataset was re-created based on findings from a double-blind, placebo-controlled, randomized, factorial trial. Bell's Palsy is characterised by a sudden, temporary weakness or paralysis of the facial muscles, commonly linked to inflammation of the controlling nerve, potentially triggered by a viral infection. The original trial focused on comparing the effects of prednisolone, acyclovir, both agents combined, or a placebo, administered over 10 days, with patient recruitment occurring within 72 hours of symptom onset. The primary measure of recovery is documented using the House–Brackmann scale.
Columns
The dataset includes 12 columns detailing patient characteristics, treatment allocation, and outcome scores:
- Patient ID: A unique identifier for each participant, with a mean value of 248.
- Sex: Records whether the patient is Male (52%) or Female (48%).
- Age: Patient age, ranging from 16 to 90, with a mean age of 44.9 years.
- Baseline Score on House–Brackmann scale: The facial function score recorded at diagnosis, which has a mean of 3.68 (range 2 to 6).
- Time between onset of symptoms and start of treatment: Categorises the time lag before treatment initiation, with 50% of patients starting within 24 hours.
- Treatment Group: Defines the specific combination of agents assigned based on the randomised 2 x 2 factorial design. Acyclovir–Prednisolone and Prednisolone–Placebo are two groups listed.
- Received Prednisolone: A boolean indicating if the patient received prednisolone (51% true).
- Received Acyclovir: A boolean indicating if the patient received acyclovir (50% true).
- 3-Month Score on House–Brackmann scale: The outcome score recorded three months after diagnosis. The mean score is 1.34 (range 1 to 4).
- Full Recovery in 3 Months: A boolean indicating full recovery at three months (72% true).
- 9-Month Score on House–Brackmann scale: The final outcome score recorded nine months after diagnosis. The mean score is 1.14 (range 1 to 4).
- Full Recovery in 9 Months: A boolean indicating full recovery at nine months (89% true).
Distribution
The data is available in a CSV file format named Bells Palsy Clinical Trial.csv, with a file size of 35.08 kB. It contains 494 valid records. The expected update frequency for this specific patient-level data extract is never.
Usage
This data is ideal for analysing the effectiveness of corticosteroids and antiviral agents as treatments for facial paralysis associated with Bell's Palsy. It can be used for building predictive models of long-term facial function recovery based on baseline severity and treatment protocol. It is also suitable for educational purposes related to clinical trial methodology, particularly randomised factorial trial designs.
Coverage
The data covers 494 patients, featuring demographic details such as Sex (nearly equal distribution) and Age (ranging from 16 to 90). The clinical data focuses on initial diagnosis scores and recovery outcomes assessed at the three-month and nine-month marks. All patients included were recruited quickly, within 72 hours following the onset of their symptoms.
License
CC0: Public Domain
Who Can Use It
- Epidemiologists and Public Health Analysts: To study the prevalence and outcomes related to facial paralysis conditions.
- Pharmaceutical Researchers: To benchmark or compare the efficacy of drug combinations in neurological conditions.
- Students of Biostatistics: For practicing analysis on real-world medical trial data sets, including analysis of boolean outcome variables.
- Data Scientists: For developing machine learning models to predict patient recovery trajectory.
Dataset Name Suggestions
- Bell's Palsy Treatment Efficacy Data
- Facial Paralysis Clinical Trial Results
- Prednisolone Acyclovir Factorial Study
- Patient Outcomes for Bell's Palsy
Attributes
Original Data Source: Facial Paralysis Clinical Trial Results
Loading...
